Tesamorelin (10MG)
$139.99
Shipping Calculated at checkout.
Out of stock
Email when stock available
FREE SHIPPING
99%+ PURITY
MADE IN USA

What is Tesamorelin (10MG)?

Chemical Structure of Tesamorelin (10MG)
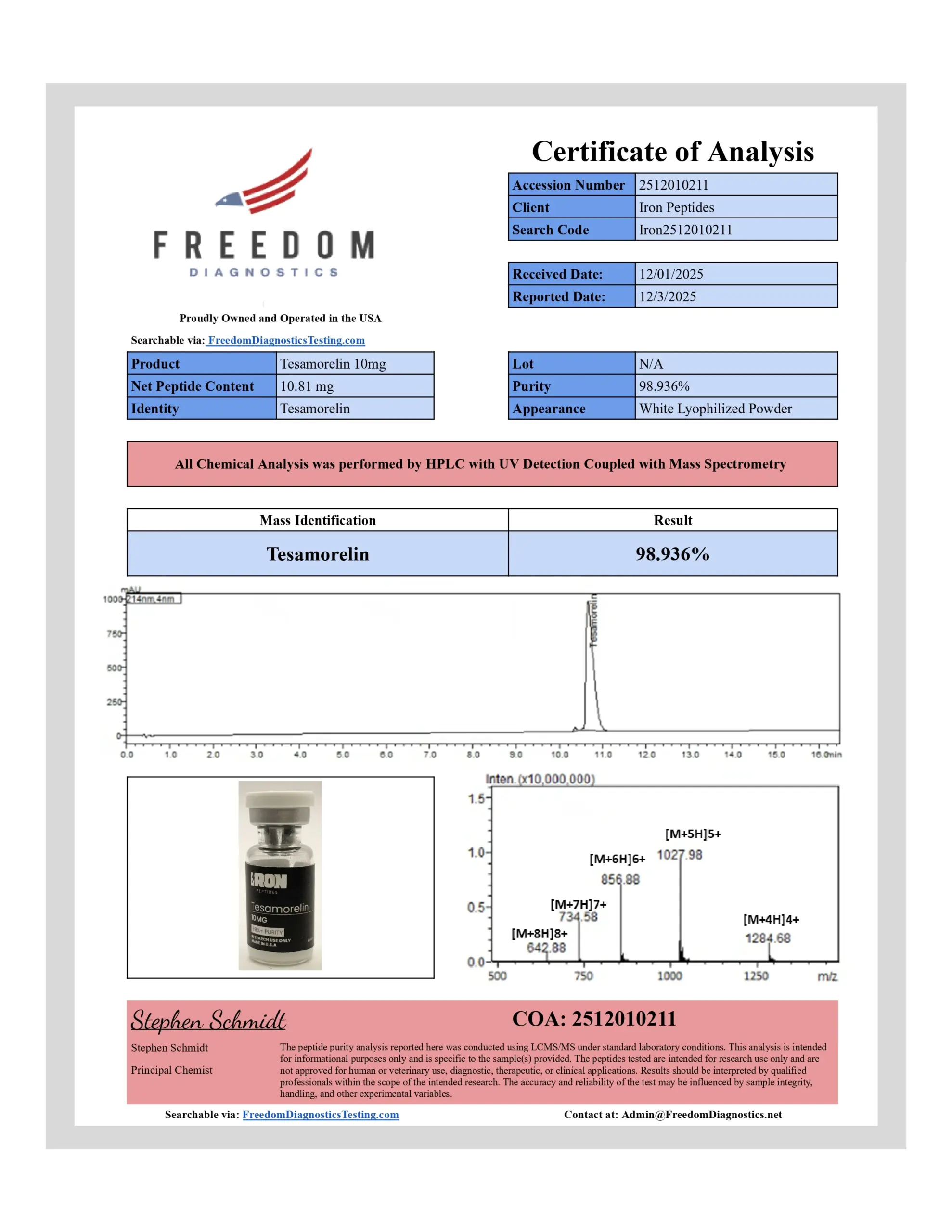
Tesamorelin comprises the 44–residue GHRH peptide sequence with a trans‑3‑hexenoic acid moiety attached to the N‑terminal tyrosine. Its molecular formula is approximately C₂₂₁H₃₆₆N₇₂O₆₇S, and molecular weight is around 5,136 Da, as recorded in PubChem (CID 146681838).
What Are the Effects of Tesamorelin (10MG)?
Endogenous GH–IGF-1 Axis Activation:
Tesamorelin binds pituitary GHRH receptors, leading to physiological GH secretion and subsequent IGF‑1 synthesis; this activation preserves natural feedback loops while avoiding supraphysiologic GH surges.
Mitochondrial Function & Bioenergetics:
In obese subjects, a 12‑month treatment correlating increases in IGF‑1 with improved phosphocreatine recovery, indicating better mitochondrial oxidative capacity.
Visceral Fat Reduction & Metabolic Modulation:
Clinical trials demonstrate 15–20% reductions in visceral adipose tissue over 26–52 weeks, along with enhanced lipid metabolism and liver fat clearance.
Insulin Sensitivity & Lipid Homeostasis:
Tesamorelin has been shown to improve glucose handling and lower triglyceride levels in individuals with fat accumulation syndromes.
Expanded Research Applications:
Current investigation extends its use to study cognitive aging, sarcopenia, neuroprotection, and metabolic resilience in preclinical and clinical models.
Citations
- PubChem Compound Summary for Tesamorelin (CID 146681838): molecular formula C₂₂₁H₃₆₆N₇₂O₆₇S; MW 5,136 Da.
- Peptides.guide entry on Tesamorelin: 44-amino-acid GHRH analogue with trans‑3‑hexenoic acid N‑terminus.
- Research Peptides summary: tesamorelin researched for GH and IGF‑1 stimulation, fat metabolism, muscle growth.
- C. Grinspoon et al. “The Effects of Tesamorelin on Phosphocreatine Recovery in Obese Subjects With Reduced GH.” J Clin Endocrinol Metab. 2014;99(1):338–343. PMID: 24142878.
- J. Falutz et al. “Effects of tesamorelin (TH9507), a growth hormone–releasing factor analog, in HIV‑infected patients with excess abdominal fat: a pooled analysis of two phase 3 trials.” J Clin Endocrinol Metab. 2010;95(9):4291–4304. PMID: 20656618.
 Tesamorelin (10MG)
Tesamorelin (10MG)
| 5 star | 0% | |
| 4 star | 0% | |
| 3 star | 0% | |
| 2 star | 0% | |
| 1 star | 0% |
Sorry, no reviews match your current selections

TrustScore 4.6
DH
Drew Hahn
2025-09-09
I decided to try something new with…
I decided to try something new with Iron Peptides, and it has already been very beneficial. Since starting, I’ve noticed many positive effects. I feel motivated to continue, and I’m confident I’ve found the right company to trust on my personal journey—Iron Peptides.
y
yari
2025-09-07
The products
The products, services and information is exceptional

Karla Rodriguez
2025-09-07
Fist time trying peptides to see what…
Fist time trying peptides to see what all the hype was about. And. I AM HOOKED! Have never felt. Better. 5 months after my neck surgery and I feel amazing. Recovery was easy and I have all my strength back!!